Developing the Science Basis for Understanding Polymer Encapsulant Degradation Mechanisms
DuraMAT uses a combined approach involving experimental aging and computational simulations to identify degradation species and quantify the factors that initiate and drive degradation.
As we address degradation at the molecular scale, our work supports experimental results that elucidate observed phenomena through an atomistic lens.
Mapping the diffusion of small-molecule contaminants in various ethylene-vinyl acetate (EVA) microstructures reveals the importance of fully curing modules and uncovers a secondary plasticization effect between acetic acid and water.
A predictive materials-based understanding of degradation in polymeric encapsulants is critical for achieving a 50-year lifespan for solar PV modules. Novel encapsulant materials are emerging, and the market share is heading toward increasingly cost-effective choices. In most cases, a molecular-scale understanding of the chemical degradation of these new materials is lacking.
Many factors, including chemical kinetics and diffusion of reaction products, can simultaneously drive the aging process. A more complete understanding of the heterogeneous degradation of encapsulants requires knowledge of the migration of species, their concentration dependence, and their combined effect on degradation rates.
Core Objective
Multi-Scale, Multi-Physics Model
Team Members
Mark Wilson, Jennifer Braid, Michael Chandross, Hannah Dedmon, Samantha Kruse, and Jessica Kustas at Sandia National Laboratories
Impact
To tackle concerns about polymeric degradation, we performed classical molecular dynamics simulations of various ethylene-vinyl acetate (EVA) systems, accounting for variables such as the percentage of vinyl acetate monomer, molecular weight, and degree of cure. This allows us to relate relevant manufacturing benchmarks like glass transition temperature to the effects of contaminants—specifically, their diffusion. The high-impact result of this research will be a deeper understanding of how combined material and environmental factors, such as incomplete curing and specific contaminant ingress, drive degradation processes that can be detected at the engineering scale.
Learn More
Dedmon, Hannah et al. 2024. “Predicting Molecular Scale Dynamics and Kinetics Occurring During Encapsulant Degradation.” Poster presented at Foundations of Molecular Modeling and Simulations (FOMMS) Conference, Snowbird, Utah, July 28–August 1, 2024. SAND2024-09533C.
Dedmon, Hannah et al. 2024. “Understanding Polymer Encapsulant Degradation: A Scale Bridging Computational Frameworks.” Presented at DuraMAT FY24 Workshop and Program Review, Berkeley, California, September 17–18, 2024. SAND2024-12692C.
Contact
To learn more about this project, contact Mark Wilson, Sandia.
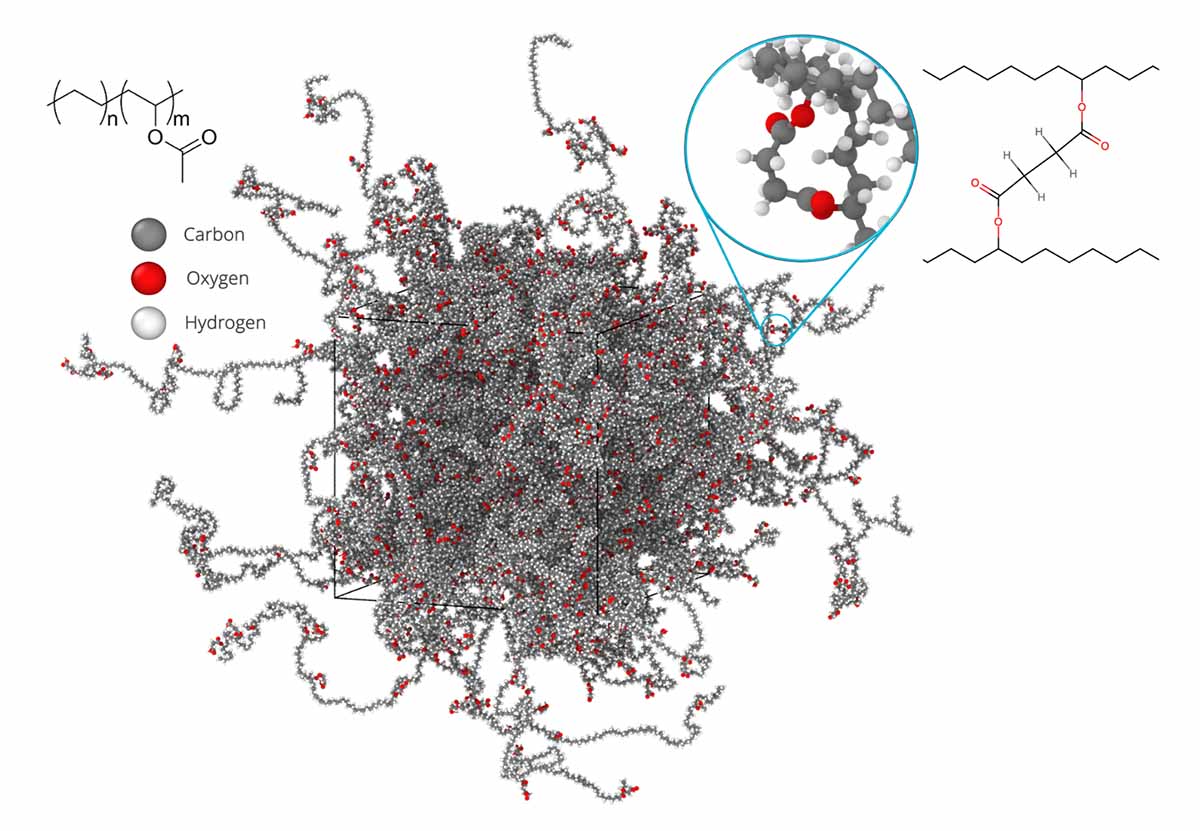
Molecular-scale model of traditional EVA encapsulant material, used to quantify diffusion and reaction kinetics occurring during degradation. Emphasis is given to the formed cross-link between acetyl groups of EVA chains, which occurs during curing. Image from Hannah Dedmon, Sandia
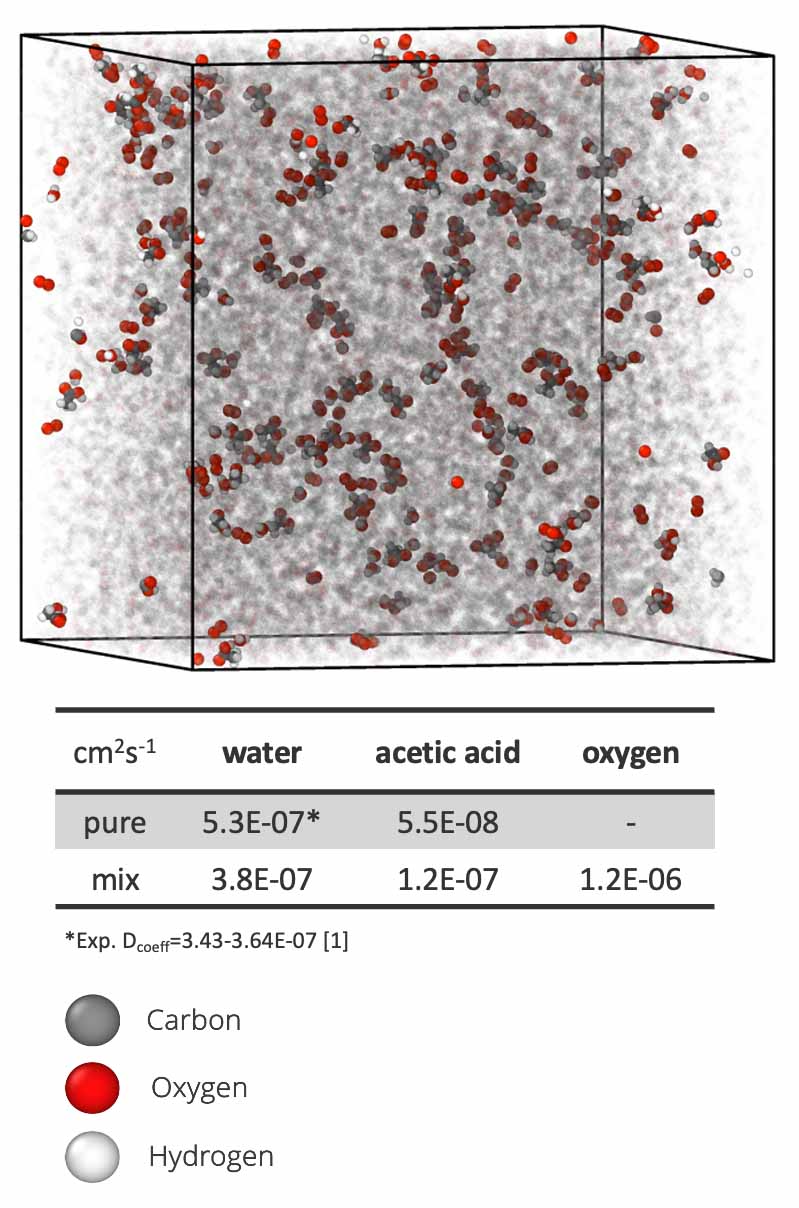
Molecular model of EVA (translucent) with small-molecule contaminants: acetic acid, oxygen, and water. Comparing the diffusion of acetic acid as a pure solvent with water and oxygen in EVA reveals an increase in the mobility of acetic acid in the presence of moisture. On the other hand, the diffusion of water decreases as expected, revealing a secondary plasticization effect. Image from Hannah Dedmon, Sandia